research
Retrograde labeling of oxytocin receptor expressing neurons
Research in the Dölen lab focuses on how the brain enables social behaviors through basic neurobiological processes such as neuromodulation and synaptic plasticity. In addition, we are interested in understanding the pathophysiology of autism and schizophrenia, disorders characterized by profound social and cognitive impairments, with the ultimate goal of designing mechanism-based therapies. Using a combination of well-established, cutting edge, and evolving techniques, our goal is to approach the daunting complexity of the brain armed with molecular, biochemical, optogenetic, electrophysiological, and behavioral strategies to dissect the biological basis of social behavior.

Photo © Jean-Louis Klein & Marie-Luce Hubert
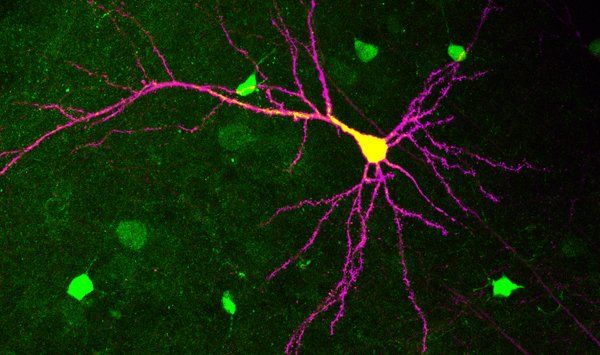
Social behaviors have evolved in species as diverse as colony forming honey bees, schooling tuna fish, pair-bonding penguins, pack wolves, as well as most primates, including humans. For these species, social behaviors are adaptive, yet nevertheless often come at some cost to the individual. Accordingly, it has been suggested that reinforcement of social interactions by the brain’s mesocorticolimbic (MCL) reward system might be required for the evolutionary persistence of these behaviors. While synaptic changes in the MCL reward system have been implicated in drug addiction, relatively little is known about how ethologically relevant “natural” rewards, such as social interaction, are encoded by this circuit. Modern optogenetic technologies afford us the ability to stimulate molecularly isolated input projections independently; when combined with whole-cell patch clamp recording, genetic manipulation, and behavioral assays, we are able to parse the synaptic and circuit elements required for social reward.
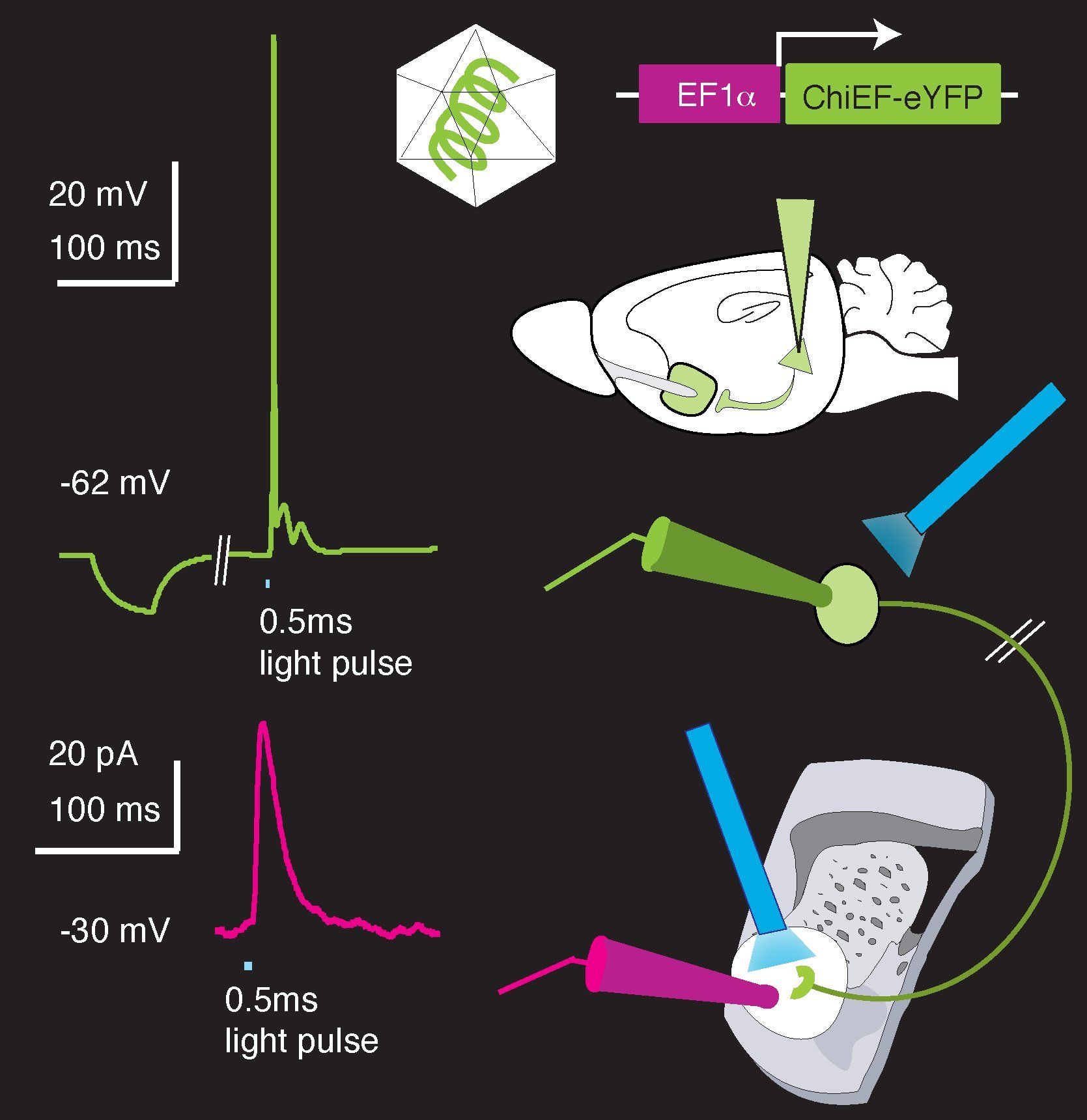
Patch clamp electrophysiology and optogenetic input stimulation
Oxytocin (OT) and arginine-vasopressin (AVP) are evolutionarily related peptides which powerfully influence a spectrum of social behaviors including parental care, conjugal and consociate affiliation, aggression, and social recognition across a large diversity of species. These peptides are primarily synthesized and released by discrete populations of cells within the hypothalamus. The Dölen lab is studying the synaptic and circuit effects of these peptides, with a focus on autism relevant functions.
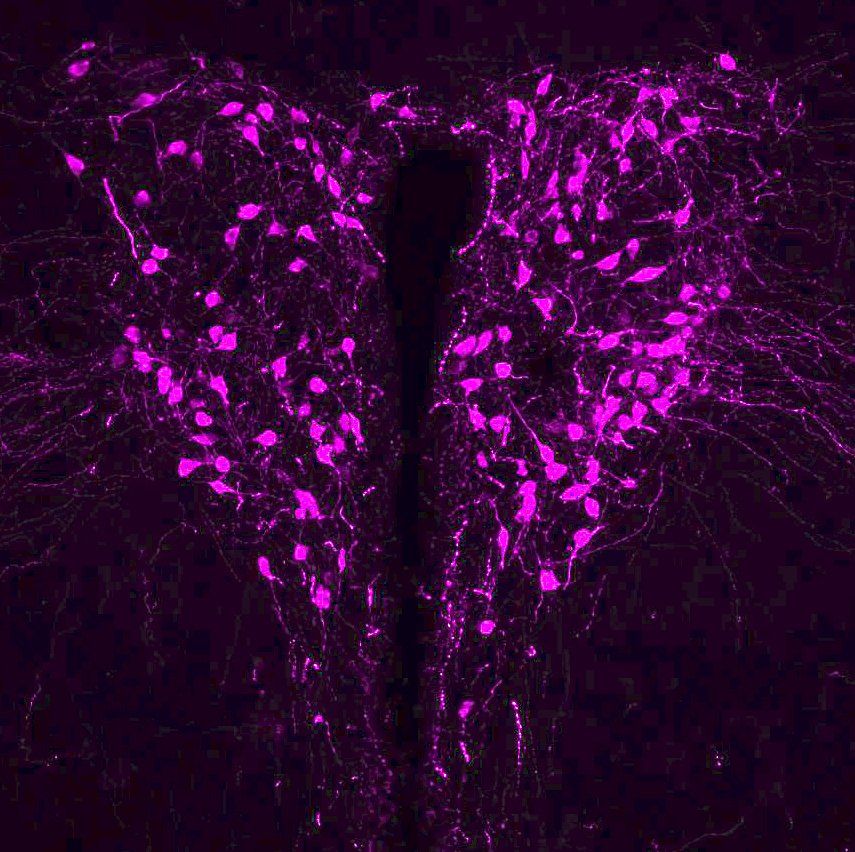
Oxytocin (OT) producing neurons in the hypothalamus
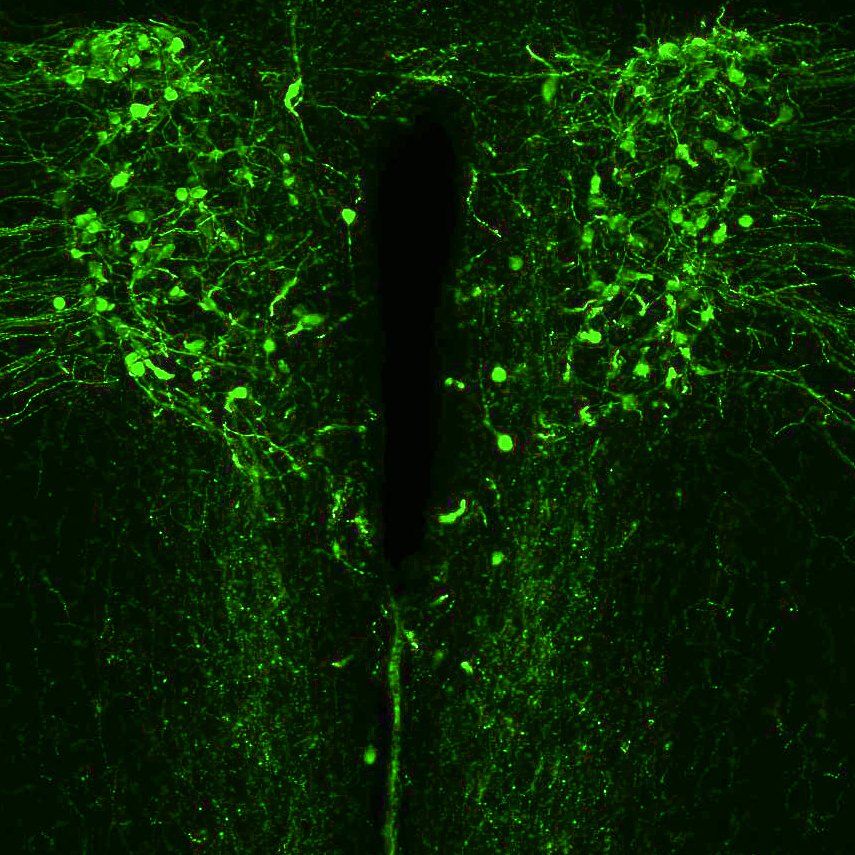
Arginine Vasopressin (AVP) producing neurons in the hypothalamus
‘Autism,’ as first described by Leo Kanner in 1943, is a developmental neuropsychiatric disorder characterized by a profound lack of interest in others. Perhaps the most straightforward hypothesis about how this ‘autistic aloneness’ develops, is that for people with autism, social interactions are simply not rewarding, or, are in some way abnormally rewarding. The Dölen lab focuses on testing this possibility in mouse models of autism. Guided by our mechanistic insights into the synaptic and circuit mechanisms of social reward, we are examining if and how autism genes, when disrupted, lead to aberrant synaptic plasticity, neuromodulation, and neuropeptide regulation across the MCL social reward circuit.

Photo: Thomas Kleindinst
We are studying the evolutionary basis of social behaviors. Octopuses and humans are separated by 540 million years of evolution. The anatomical organization of their brains is completely different from ours. Using whole genome sequencing data from Octopus bimaculoides
(pictured above) we are able to determine the extent to which, despite these differences, the molecular building blocks for complex behaviors are shared across lineages. In addition, we are sequencing the genome of two sister species of octopus, the Larger Pacific Striped Octopus (scientific name pending) and the Pygmy zebra octopus (Octopus chierchiae). This genomic data will enable phylogenetic comparisons that we hope will allow us to understand how sociality evolved.
